This article was published on 9 May 2025 in the journal Frontiers in Veterinary Science and its rate of sharing and views shows that it is gaining a lot of interest. I am copying the article here, with attribution, to ensure that dog owners make an informed choice when the drug (known as Beransa in our local market) is recommended.
There is clinical evidence that osteoarthritis worsens at an increased rate when using the medication.
One of the authors, Dr Mike Farrell, has also published this lecture to veterinary professionals, to explain the trial process and the history of adverse effects of this class of drugs.
I’m a science geek and I like to understand clinical trial information and the defensibility of clinical trial information. I encourage my clients to be knowledgeable and to ask the right questions of their vet when all medications are prescribed for their dogs. This post, like others about this drug, is shared to inform dog owners.
Objectives: To conduct a specialist-led disproportionality analysis of musculoskeletal adverse event reports (MSAERs) in dogs treated with bedinvetmab (Librela™) compared to six comparator drugs with the same indication. Furthermore, to report the findings from a subset of dogs whose adverse event (AE) data underwent independent adjudication by an expert panel.
Study design: Case–control study and case series analysis.
Sample population: The European Medicines Agency’s EudraVigilance database (2004–2024) and 19 client-owned dogs.
Methods: An EBVS® Veterinary Specialist in Surgery individually reviewed all MSAERs to Librela™, Rimadyl®, Metacam®, Previcox®, Onsior®, Galliprant®, and Daxocox® (2004–2024). The primary null hypothesis was that Librela’s MSAER rate would not exceed that of comparator drugs by more than 50%. The secondary hypothesis was that MSAER would surge and taper following the launch of new drugs.
Results: The disproportionality analysis did not support the hypotheses. Ligament/tendon injury, polyarthritis, fracture, musculoskeletal neoplasia, and septic arthritis were reported ~9-times more frequently in Librela-treated dogs than the combined total of dogs treated with the comparator drugs. A review of 19 suspected musculoskeletal adverse events (MSAEs) by an 18-member expert panel unanimously concluded a strong suspicion of a causal association between bedinvetmab and accelerated joint destruction.
Conclusion: This study supports recent FDA analyses by demonstrating an increased reporting rate of musculoskeletal adverse events in dogs treated with Librela. Further investigation and close clinical monitoring of treated dogs are warranted.
Impact: Our findings should serve as a catalyst for large-scale investigations into bedinvetmab’s risks and pharmacovigilance.
1 Introduction
Osteoarthritis (OA) is the most prevalent chronic pain condition in companion animals, and is a significant contributor to reduced quality of life and premature death (1). Although a diverse array of therapeutic approaches are currently available, all possess limitations, including suboptimal efficacy and the potential for severe adverse reactions. Chronic pain management is challenged by the trade-off between safety and efficacy. Analgesic drugs that provide significant pain relief can carry a higher risk of adverse reactions than safer, less effective options like glucosamine-chondroitin joint supplements (2). This therapeutic dilemma is complicated by differing risk perceptions between veterinarians and caregivers. Veterinarians, due to their medical training, may be more comfortable with the risks associated with prescription analgesics, whereas caregivers may be more hesitant, sometimes declining or limiting their use even when deemed necessary by the veterinarian (3). This disconnect is underscored by a 2018 study revealing that 22% of dogs for whom analgesics were recommended by their veterinarians did not receive them (4).
Development of new therapies offering enhanced safety and efficacy can help bridge the gap between veterinary recommendations and caregiver acceptance. Bedinvetmab (Librela™), a monoclonal antibody (mAb) targeting nerve growth factor (NGF), represents a significant advancement in canine osteoarthritis (OA) pain management. Following its approval by the European Commission in November 2020, it became the first mAb authorized for this indication. The Food and Drug Administration (FDA) granted U.S. marketing authorization 2.5 years later, and the Australian Pesticides and Veterinary Medicines Authority subsequently registered the same drug as Beransa™.
While these regulatory approvals underscored worldwide confidence in veterinary mAbs, their human equivalent were associated with substantial safety concerns. Specifically, NGF modulates bone and cartilage turnover (5), and its inhibition is linked to accelerated joint degeneration in humans (6, 7). This was evidenced in 2012, when clinical trials of anti-NGF mAbs (aNGFmAbs) revealed rapidly progressive osteoarthritis (RPOA) (8), leading the FDA to impose a two-year clinical trial hold and mandate a risk evaluation and mitigation strategy (REMS) post-hold. However, even with stringent screening, low dosing, and NSAID prohibition after the REMS was implemented, RPOA risk persisted (9–11). While the exact mechanism is still under investigation, human clinical trials did not support the hypothesis that RPOA is caused by overuse of weight-bearing joints (7, 10).
Post-marketing pharmacovigilance is crucial for continuously monitoring a drug’s safety and efficacy after it enters the market, as clinical trials cannot capture the full spectrum of potential adverse reactions. It involves a combination of voluntary reporting of adverse drug reactions (ADRs) by healthcare professionals and the public, and proactive surveillance programs, including government-funded, industry-sponsored, and independent research. Their findings inform regulatory decisions, which can range from label updates and limited use restrictions to, in rare cases, market withdrawal if the risks outweigh the benefits (12).
When an animal exhibits unexpected clinical signs following drug administration, differentiating these effects from the underlying disease or a new unrelated condition can be complex. Nevertheless, prompt identification of potential causal relationships is paramount for ensuring patient safety. The thalidomide tragedy exemplifies the critical importance of rigorous pre-clinical and post-marketing drug safety surveillance. Insufficient testing and a lack of robust post-marketing surveillance systems failed to identify the teratogenic potential of thalidomide (13). This resulted in widespread use of the drug leading to severe birth defects in thousands of children, highlighting the potentially devastating consequences of delayed recognition of drug-related adverse events.
In December 2024, the FDA issued an open letter to veterinarians, alerting them to neurological and musculoskeletal safety signals identified during their post-marketing surveillance of Librela (14). The FDA’s Center for Veterinary Medicine (CVM) employed an algorithmic approach to evaluate ADRs. Their approach, incorporating disproportionality analysis, statistically assessed the frequency of reported adverse events (AEs) in dogs receiving Librela compared to those treated with other OA medications. The FDA’s analysis identified 18 distinct safety signals in dogs administered Librela, encompassing neurological events, urinary problems, and musculoskeletal disorders (14). Notably, the FDA observed a disproportionately elevated reporting rate of “lameness” in dogs receiving Librela. In response, the Center for Veterinary Medicine (CVM) advised veterinarians to proactively inform pet owners about these potential adverse reactions (14).
The CVM emphasized that their objective was to generate hypotheses, acknowledging the inherent limitations of establishing definitive causality (14). They noted that AE reporting systems are subject to various biases, including underreporting, reporting biases influenced by [social] media attention, and confounding factors such as concomitant medications. Furthermore, the CVM’s reliance on algorithmic analyses of secondary data, without the benefit of expert clinical interpretation, introduces additional diagnostic uncertainty (15). To address this limitation, we employed a two-pronged approach. Firstly, we conducted a specialist-led disproportionality analysis of musculoskeletal adverse event reports (MSAERs) to expand upon the CVM’s work. This analysis tested the null hypothesis that Librela’s MSAER rate would not exceed that of six comparator drugs with the same indication by more than 50%. Secondly, we report the findings from a subset of dogs whose AE data were subjected to independent adjudication by an expert panel and subsequently submitted to the European Medicines Agency (EMA).
2 Materials and methods
2.1 MSAER disproportionality analysis
A detailed description of the EudraVigilance database (EVD) analysis is provided in Supplementary Video 1. Briefly, accurate identification of clinically relevant musculoskeletal adverse events (MSAEs) required a thorough understanding of the pharmacovigilance system’s information flow (Figure 1), and the limitations inherent in the system. Specifically, diagnostic terms submitted by primary reporters are only published if they are listed in the Veterinary Dictionary for Drug Regulatory Activities (VeDDRA). Otherwise, the Marketing Authorization Holder (MAH; Zoetis, Louvain-la-Neuve, Belgium) selects a diagnostic term from a predefined list including clinical signs (e.g., lameness), non-specific diagnoses (e.g., arthritis), and specific diagnoses (e.g., ligament rupture) (16). At the time of analysis, VeDDRA contained 113 musculoskeletal and 313 neurological AE diagnoses (i.e., low-level terms, LLTs) (16). Many LLTs exhibit clinically relevant overlap (Table 1). For example, “limb weakness” (a musculoskeletal LLT) may indicate a neurological problem, while “collapse of leg” (a neurological LLT) might describe an orthopedic AE. With over 35,000 possible combinations of musculoskeletal and neurological LLTs, a simple algorithmic approach was not considered feasible.
To ensure consistency and account for the complex clinical judgments required for data interpretation, a single EBVS® Specialist in Surgery (Author 1) reviewed musculoskeletal adverse event reports (MSAERs) logged within the European Medicines Agency’s (EMA) EudraVigilance database (EVD) from its 2004 inception to December 31, 2024. A descriptive disproportionality analysis was used to compare the incidence of MSAERs associated with Librela to those of six other veterinary analgesics: Rimadyl®, Metacam®, Previcox®, Onsior®, Galliprant®, and Daxocox®. This analysis aimed to identify any potential temporal trends in MSAER reporting, particularly following the introduction of new medications. All adverse event reports (AERs) are filed in the EVD under trade names; therefore, trade names are used consistently throughout this manuscript.
2.2 Case series inclusion criteria
This study utilized a retrospective, case series design. Case recruitment was initiated by Author 1 following the observation of a suspected case of RPOA in a dog receiving Librela. This case was shared on a specialist veterinary forum (17). Subsequently, over an 11-month period, multiple clinicians who subscribed to the forum contacted Author 1 to share concerns regarding serious MSAEs in dogs treated with Librela. Based on these communications, an independent working group was formed comprising clinicians with firsthand experience with MSAEs (see below). The primary objective was to investigate a potential association between Librela administration and the observed pathology. Given the lack of prior records of RPOA in dogs, the working group sought advice from an expert in human neuro-osteoarthropathy (Author 2) and two EBVS® Specialists in Diagnostic Imaging with published expertise in musculoskeletal imaging (Authors 3 and 4).
Twenty-three suspected musculoskeletal adverse events (MSAEs) were independently reviewed by nine investigators with a combined 128 years of experience in referral practice (Authors 1, 5, 6, 7, 9, 11, 14, 15, 16). Clinical data from each case, including signalment, clinical signs, Librela dosing information, concurrent medications, treatment outcomes, and relevant diagnostic test results (radiographs, CT scans, synovial fluid analysis, histopathology), were evaluated. Four cases were excluded from further analysis due to incomplete data or insufficient evidence to support a causal relationship. Twelve MSAERs had already been filed in the EVD, and retrospective reports were filed for the remaining cases at this time.
2.3 Case series adjudication
The independent adjudication panel comprised 12 veterinary orthopedic surgeons, an orthopedic consultant specializing in human neuro-osteoarthropathy, two veterinary diagnostic imaging specialists, two veterinary anesthetists, and a cancer researcher with expertise in monoclonal receptor-based therapeutics. The adjudication panel demonstrated collective expertise including 157 relevant peer-reviewed publications spanning monoclonal antibodies, neuropathic arthropathy, canine osteoarthritis (OA), pathological fractures, and humeral intracondylar fissure (HIF).
Transcripts from the 2012 and 2021 Arthritis Advisory Committee (AAC) and Drug Safety and Risk Management Advisory Committee Meetings were reviewed. Our analysis focused on their joint safety review of humanized anti-nerve growth factor monoclonal antibodies (aNGFmAbs) (8–10). The following limitations in clinical trials used to define human RPOA were acknowledged:
1. Inconsistent baseline imaging: Humans enrolled in low-back pain aNGFmAb clinical trials who developed RPOA did not undergo baseline radiographic imaging of the affected joint (s) before starting treatment (8, 18).
2. Nonspecific terminology: The definition of human RPOA included joint pathology “falling well outside the natural history of OA” (8). Notably, this criterion lacked a specific definition of the “natural history of OA” and did not reference a control group with typical OA progression.
3. Inapplicability of the human definition: The specific term “RPOA” was not adopted for our case adjudication due to its reliance on measurements of large human joints using standing radiographs or MRI (11).
Nineteen dogs with suspected MSAEs following bedinvetmab treatment were independently evaluated by Authors 3 and 4. Suspected drug-related AEs were defined according to the AAC’s methodology as joint pathology “falling well outside the natural history of OA” (8). This included pathological fractures or luxations in osteoarthritic joints, and subchondral osteolysis in the absence of clinical evidence of septic or immune-mediated arthritis. Inter-rater agreement for the two diagnostic imaging specialists was tested using the Fleiss κ coefficient (19).
Diagnostic images were formatted and annotated by Authors 1 and 3. Subsequently, all 18 experts independently reviewed the annotated images, emulating standard clinical practice. The entire cohort of 19 cases was evaluated collectively by the adjudication panel to determine potential drug causality, rather than assessing each case individually, mirroring the AAC’s 2012 protocol (8). Readers can review the cases by watching Supplementary Video 2.
A three-tiered system was used to describe a potential causal relationship between bedinvetmab treatment and MSAEs. Outcomes explicitly implying a known causal link (e.g., “definitely related”) were avoided to reflect the inherent uncertainty of this assessment. Instead, the experts described their personal judgment as “very suspicious,” “suspicious,” or “insufficient evidence” of a potential causal relationship.
2.4 AER translation errors
Translation errors were identified by comparing MSAERs submitted by attending veterinarians with corresponding reports filed by the MAH. A clinically relevant discrepancy between the reports was considered a translation error.
3 Results
3.1 MSAER disproportionality analysis
A total of 4,746 MSAERs were identified between May 20, 2021 (3 months after Librela’s European release) and December 31, 2024. Following the exclusion of 457 comparator medication reports which specified co-administration of Librela, 4,289 MSAERs remained. Of these, 3,755 (87.5%) were attributed to Librela. The majority of MSAERs (3,411, 79.5%) were excluded due to confounding neurological and/or systemic/neoplastic diagnoses (Supplementary Video 1), resulting in a final cohort of 878 eligible MSAERs for analysis, with 789 (90%) attributed to Librela (Supplementary Table S1). Most primary reports to Librela (88%) were submitted by veterinarians and other healthcare professionals (Figure 1).
Ligament/tendon injury, polyarthritis, fracture, musculoskeletal neoplasia, and septic arthritis were reported ~9-times more frequently in Librela-treated dogs than the combined total of dogs treated with the comparator medications (Figure 2). Furthermore, accumulated MSAERs for Librela over 45 months exceeded those of the highest-ranking NSAID (Rimadyl) by ~20-fold and surpassed the combined accumulated MSAERs of all comparator drugs over 240 months by ~3-fold (Figure 3). These findings did not support the null hypothesis that Librela’s MSAER rate would not exceed that of comparator drugs by more than 50%. Moreover, the secondary hypothesis that MSAER would surge and taper following the launch of new drugs was not supported (Figure 3).
3.2 Specific diagnoses and outcomes for Librela’s MSAERs
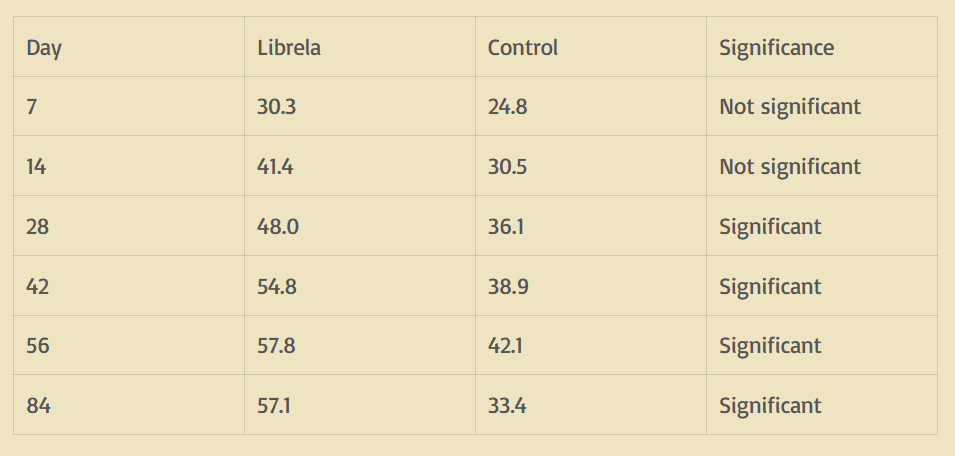
The most frequent diagnostic terms selected by the MAH were “arthritis” or associated clinical signs (e.g., “lameness”, “joint pain”, “difficulty climbing stairs”), encompassing 530 cases (67%) (Figure 4). Of these, the MAH filed 442 reports (83.4%) as “not serious”. The remaining 259 ADRs included ligament injuries, limb collapse, polyarthritis, bone cancer, and fractures. Among these, the MAH filed 138 reports (52.3%) as “not serious”.

The most frequently reported outcome was “unknown” (310 dogs; 39%). Of the remaining dogs, 177 (22%) experienced AEs that were reported as “recovered/resolving/normal”; 229 (29%) were filed as “ongoing”; 15 (2%) “recovered with sequelae”; and 63 dogs (8%) died or had been euthanized.
3.3 Case series adjudication outcome
Clinical and radiographic characteristics are summarized in Table 2 and Figures 5–13. Mean ± SD number of Librela doses was 12.7 ± 9.5 (range 1–30), with a dose range of 0.4–0.76 mg/kg (mean 0.62 ± 0.08 mg/kg). Referral for investigation of suspected RPOA was made at least 6 months after Librela initiation in 13/19 cases. Eleven dogs (58%) received regular concurrent NSAIDs. The most frequently affected joint was the elbow (13/19 dogs, 68%), followed by the stifle and hock (two dogs each), and hip (one dog). Seven dogs (37%) sustained pathological fractures, and two (10.5%) had joint luxations. Two dogs with clinically normal hock joints before initiating Librela therapy developed severe non-index hock joint destruction after Librela treatment for elbow OA.
Histopathological examination of bone and synovial tissue from four dogs revealed no evidence of inflammatory arthropathy, tick-borne diseases, or neoplasia. A pathologist who was invited to compare their findings to those reported in a submitted article on human RPOA (20) commented that the pathological features were similar (21).
Interobserver agreement between diagnostic imaging specialists was substantial (κ = 0.68, 95% CI 0.4–0.97). Both specialists were very suspicious of a potential causal relationship between the observed pathology and Librela treatment in 68% of dogs (13/19). Furthermore, all 18 panelists (including the two diagnostic imagers) were very suspicious of a potential causal relationship between Librela treatment and the observed pathology.
3.4 AER translation errors
Translation errors were identified in 9/19 cases (52%) (Table 2 and Supplementary Figures S1–S6). They included incorrect diagnoses (n = 5), severity (n = 5), and outcome (n = 5). Furthermore, the MAH reported two cases as “overdoses”, despite the administered dosages falling within the recommended range.
4 Discussion
This study reveals a striking disparity in musculoskeletal adverse event reports to Librela compared to six comparator drugs. Ligament/tendon injuries, polyarthritis, fractures, musculoskeletal neoplasia, and septic arthritis were reported nine times more frequently in Librela-treated dogs. Worryingly, since its European release, Librela has accumulated 20 times more reports than the highest-ranking comparator drug (Rimadyl) and three times more than all comparator drugs combined over a 20-year period. Furthermore, independent expert review of a subset of cases strongly supported a causal association between Librela and accelerated joint destruction.
Librela experienced rapid market penetration following its 2021 European release. Zoetis recently reported global distribution exceeding 21 million doses, translating to an estimated average daily distribution of over 15,000 doses (22). This initial market success has been tempered by emerging concerns regarding bedinvetmab’s safety. These concerns have been amplified by various factors, including the FDA’s safety update (14), negative press coverage (23), the European Commission’s investigation into potential anticompetitive conduct by Zoetis (24), and the emergence of online communities disseminating safety concerns. This confluence of events has fostered a climate of apprehension and confusion. Addressing these concerns requires unbiased and rigorous post-marketing pharmacovigilance to evaluate this drug’s true risk–benefit profile.
Assessing the “expectedness” of adverse drug reactions (ADRs) is fundamental to effective pharmacovigilance. In causal relationship investigations, statisticians use Bayesian principles to evaluate reaction likelihood, considering plausibility and prior knowledge (15). The FDA’s ABON (Algorithm for Bayesian Onset of symptoms) links drug exposure to adverse events AEs (15). For example, when applied to NSAIDs, ABON incorporates prior knowledge of prostaglandin inhibition, its effect on gastrointestinal (GI) mucosal integrity, and the established link between NSAIDs and GI ulceration (25). Notably, NSAIDs can cause subclinical GI damage, undetectable without endoscopy (25). When clinical signs occur, vomiting and diarrhea are common manifestations (26). However, the FDA does not use sales-figure-based prevalence estimates, because they can dramatically underrepresent true incidence (15). For example, comparing carprofen’s AERs to drug sales suggests vomiting and diarrhea occur in <1/10,000 doses, falsely implying that common side-effects are “very rare” (27).
The NSAID analogy is valuable for three reasons. First, while prostaglandins safeguard gastrointestinal integrity, NGF plays a similar pivotal role in bone and cartilage repair (5). Second, serious subclinical cartilage and bone degeneration often precede clinical signs (28). Third, recent claims of bedinvetmab’s “rare” or “very rare” AEs (29) are based on similar methodology to the carprofen analysis described above. Given NGF’s diverse roles and prior evidence of RPOA, subchondral bone fractures, and atraumatic joint luxations in humans and animals (8, 11, 30–33), bedinvetmab-associated MSAEs are an expected consequence of NGF inhibition.
Bayesian analysis, while powerful, can be susceptible to subjective biases. This is exemplified by the FDA’s role in the opioid crisis. Despite acknowledging the inherent risk of opioid addiction, the agency over-relied on a five-sentence letter, disproportionately cited as evidence of low addiction rates with oral opioid therapy (34). The FDA’s subsequent mischaracterization of addiction risk as “minimal” was heavily criticized (34). Similarly, the hypothesis that RPOA is a uniquely human problem has faced significant criticism. Multiple experts have contested this claim (32, 35, 36), citing weak supporting data (37). Notably, the joint safety claims outlined in Librela’s datasheet (38) are based on radiographic assessment of five healthy beagles who received the recommended dose (37). This study reported “mild” cartilage erosion in two dogs, despite erosion being, by definition, a severe form of cartilage pathology. Furthermore, despite being invited to provide annotated images to clarify this discrepancy (36), Zoetis declined to do so (39).
Janssen (fulranumab), Pfizer (tanezumab), and Regeneron (fasinumab) self-reported accelerated joint degeneration in their pre-marketing human aNGFmab clinical trials (8). The FDA responded quickly and decisively, voting 21–0 to recognize RPOA as a side-effect of aNGFmAbs and mandating a sophisticated risk mitigation strategy for all subsequent trials (8). The scale of the precautions undertaken by these pharmaceutical companies is exemplified by Pfizer’s tanezumab program, which involved 18,000 patients and 50,000 radiographs analyzed by 250 experts (11).
When viewed in context, bedinvetmab’s limited pre-marketing clinical trials raise serious concerns. Only 89 dogs received more than three doses (40), and crucially, no radiographic screening for accelerated joint degeneration was conducted (40, 41). Unlike Janssen, Pfizer, and Regeneron, Zoetis was unable to self-report accelerated joint destruction due to the absence of radiographic investigations. Consequently, we must rely on post-marketing surveillance to determine whether companion animals experience the adverse joint pathology observed in humans and laboratory animals treated with aNGFmAbs.
We initially intended to publish only the 19 adjudicated cases as a case series. However, we recognized the potential for case examples of severe pathology to be dismissed as outliers—isolated events swamped by the widespread positive experiences reported with bedinvetmab. Given Librela’s popularity, this perspective would be understandable. However, this response would be analogous to assessing the risk of NSAID-induced gastrointestinal harm by comparing the incidence of perforating gastric ulcers with NSAID sales figures. It should be obvious that such an approach neglects the critical fact that NSAIDs can induce subclinical harm which is undetectable without inconvenient tests such as endoscopy. Crucially, unlike the gastrointestinal mucosa, which possesses significant regenerative capacity, cartilage damage, once incurred, is largely irreversible (42). This fundamental difference underscores the gravity of MSAEs associated with aNGFmAbs.
To complement the FDA’s Bayesian analyses, which collected data from May 2023 to March 2024, we employed a descriptive evaluation to 20 years of MSAER data. This approach was deemed complementary because the ABON algorithm primarily focuses on identifying ADRs occurring shortly after medication initiation, while MSAEs often exhibit a long latency period between drug administration and AE detection. This hypothesis is supported by the observation that most reactions in the FDA’s analysis occurred within the first week post-injection, whereas most human RPOA (9, 10) and 13/19 cases in our study manifested at least 6 months after treatment initiation.
A limitation of our descriptive analysis is the inherent subjectivity associated with expert judgment. To mitigate potential bias, the adjudication panel primarily comprised veterinarians with a shared interest in advancing pain management for companion animals. Importantly, none had financial ties to veterinary pharmaceutical companies. Having mitigated bias, and recognizing the inherent subjectivity in data analysis and interpretation, we prioritized data presentation to facilitate independent judgment by readers, regardless of their level of expertise. Another acknowledged limitation of our study pertains to the FDA’s guidance that AE signal detection should primarily serve as a hypothesis-generating tool. Accordingly, our exploratory study was designed to identify potential safety signals rather than provide a comprehensive safety profile. As such, it cannot address specific questions like the impact of NSAID co-administration on MSAER risk. However, we believe these findings offer valuable insights and will stimulate further investigation.
Our study highlights an important weakness in the current pharmacovigilance system: the lack of comprehensive terminology for accurately capturing serious AEs. The absence of RPOA as a diagnostic term in VeDDRA is of particular concern, potentially leading to a substantial underestimation of MSAERs. Without a specific term, these events may be misclassified as manifestations of the underlying condition being treated (e.g., “arthritis” or “lameness”), obscuring the true incidence and severity of ADRs. To address this deficiency and enhance data quality, we formally requested the addition of the term “RPOA” to VeDDRA (16). Our proposed definition, “joint pathology falling well outside the natural history of OA,” leverages the expertise and clinical judgment of reporting veterinarians with direct access to patient data.
An FDA panelist involved in the adjudication of humanized aNGFmAbs eloquently summarised our current belief: “All parties agree that the use of aNGFmabs is effective, but they are associated with a unique, rapidly progressing form of OA…and we can only speculate as to its causes (8).” In animals, just as in humans, the goal of effective pain management is paramount. However, we must also ensure that our therapeutic interventions do not inadvertently exacerbate the underlying condition. To uphold the highest standard of care for companion animals, we hope to apply the same rigorous scrutiny to veterinary mAbs as was employed in human healthcare.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material.
Author contributions
MF: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. FW: Formal analysis, Investigation, Writing – review & editing. IC: Formal analysis, Investigation, Methodology, Supervision, Writing – review & editing. GS: Formal analysis, Writing – review & editing. LC: Formal analysis, Resources, Writing – review & editing. RA: Formal analysis, Supervision, Writing – review & editing. DP: Formal analysis, Supervision, Writing – review & editing. RS: Formal analysis, Writing – review & editing. DV: Formal analysis, Writing – review & editing. MA-V: Formal analysis, Resources, Writing – review & editing. AP: Formal analysis, Writing – review & editing. RQ: Formal analysis, Writing – review & editing. JH: Formal analysis, Writing – review & editing. SC: Formal analysis, Writing – review & editing. CJ: Formal analysis, Writing – review & editing. MH: Formal analysis, Writing – review & editing. AM: Formal analysis, Writing – review & editing. MG: Formal analysis, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2025.1581490/full#supplementary-material
SUPPLEMENTARY VIDEO 1 | Demonstration of the EudraVigilance database MSAER search strategy. The database can be accessed at: https://www.adrreports.eu/vet/en/search.html# (Accessed September 18–23, 2024 and January 1–10, 2025).
SUPPLEMENTARY VIDEO 2 | Narrated slideshow used by the independent adjudication panel.
SUPPLEMENTARY TABLE S1 | 789 musculoskeletal adverse event reports to Librela (May 2021–December 2024).
SUPPLEMENTARY FIGURE S1 | Case 1—AER translation error. The attending specialist filed an AER specifying their suspicion of RPOA. The MAH filed a report with an incorrect diagnosis of septic arthritis.
SUPPLEMENTARY FIGURE S2 | Case 2—AER translation error. The MAH filed a report specifying “overdose” but the administered dose (0.6 mg/kg) was in the recommended range (38).
SUPPLEMENTARY FIGURE S3 | Case 4—AER translation error. A 9.3-year-old Labrador Retriever sustained a pathological elbow fracture. The attending specialist filed an AER to the VMD specifying their suspicion of RPOA. This report was shared with the MAH, who filed a report for non-serious “arthritis”, with a listed outcome of recovered/resolving.
SUPPLEMENTARY FIGURE S4 | Case 10—AER translation error. A 10-year-old GSD required hindlimb amputation to manage erosive tarsometatarsal OA. The attending specialist filed an AER to the VMD including the result of the histopathological analysis (which was consistent with RPOA). The MAH filed a report stating that the AE was recovered/resolving following “digit amputation”.
SUPPLEMENTARY FIGURE S5 | Case 11—AER translation error. The attending specialist filed an AER to the VMD specifying a diagnosis of ongoing RPOA. The MAH filed a report for non-serious arthritis which was recovered/resolving.
SUPPLEMENTARY FIGURE S6 | Case 12—AER translation error. The attending specialist filed an AER to the VMD specifying a diagnosis of severe RPOA. The MAH filed a report for a non-severe bone and joint disorder which was recovered/resolving. The MAH filed a report specifying “overdose” but the administered dose (0.7 mg/kg) was in the recommended range (38).
SUPPLEMENTARY FIGURE S7 | Case 13—AER translation error. The attending specialist filed an AER specifying their suspicion of RPOA. The MAH filed a report with an erroneous diagnosis of osteosarcoma.
SUPPLEMENTARY FIGURE S8 | Case 15—AER translation error. A 9.5-year-old English Bull Terrier developed erosive arthritis in previously normal hock joints. The MAH filed a report with a diagnosis of non-serious swollen joint which was recovered/resolving.
SUPPLEMENTARY FIGURE S9 | Case 18—AER translation error. A 6-year-old Australian Shepherd developed bilateral stifle joint luxations and fibular fractures following 8 doses of Librela. The MAH filed an AER designating this reaction as not serious.
References
1. O’Neill, DG, Brodbelt, DC, Hodge, R, Church, DB, and Meeson, RL. Epidemiology and clinical management of elbow joint disease in dogs under primary veterinary care in the UK. Canine Genet Epidemiol. (2020) 7:1. doi: 10.1186/s40575-020-0080-5
PubMed Abstract | Crossref Full Text | Google Scholar
2. Barbeau-Gregoire, M, Otis, C, Cournoyer, A, Moreau, M, Lussier, B, and Troncy, E. A 2022 systematic review and meta-analysis of enriched therapeutic diets and nutraceuticals in canine and feline osteoarthritis. Int J Mol Sci. (2022) 23:10384. doi: 10.3390/ijms231810384
PubMed Abstract | Crossref Full Text | Google Scholar
3. Belshaw, Z, Asher, L, and Dean, RS. The attitudes of owners and veterinary professionals in the United Kingdom to the risk of adverse events associated with using non-steroidal anti-inflammatory drugs (NSAIDs) to treat dogs with osteoarthritis. Prev Vet Med. (2016) 131:121–6. doi: 10.1016/j.prevetmed.2016.07.017
PubMed Abstract | Crossref Full Text | Google Scholar
4. Anderson, KL, O’Neill, DG, Brodbelt, DC, Church, DB, Meeson, RL, Sargan, D, et al. Prevalence, duration and risk factors for appendicular osteoarthritis in a UK dog population under primary veterinary care. Sci Rep. (2018) 8:5641. doi: 10.1038/s41598-018-23940-z
PubMed Abstract | Crossref Full Text | Google Scholar
5. Fiore, M, Chaldakov, GN, and Aloe, L. Nerve growth factor as a signaling molecule for nerve cells and also for the neuroendocrine-immune systems. Annu Rev Neurosci. (2009) 20:133–45. doi: 10.1515/REVNEURO.2009.20.2.133
PubMed Abstract | Crossref Full Text | Google Scholar
6. Hochberg, MC. Serious joint-related adverse events in randomized controlled trials of anti-nerve growth factor monoclonal antibodies. Osteoarthr Cartil. (2015) 23:S18–21. doi: 10.1016/j.joca.2014.10.005
PubMed Abstract | Crossref Full Text | Google Scholar
7. Hochberg, MC, Carrino, JA, Schnitzer, TJ, Guermazi, A, Walsh, DA, White, A, et al. Long-term safety and efficacy of subcutaneous tanezumab versus nonsteroidal anti-inflammatory drugs for hip or knee osteoarthritis: a randomized trial. Arthritis Rheumatol. (2021) 73:1167–77. doi: 10.1002/art.41674
PubMed Abstract | Crossref Full Text | Google Scholar
8. Arthritis Advisory Committee (AAC). Meeting transcript. March 12, 2012. Available online at: https://web.archive.org/web/20161023214450/http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/ArthritisAdvisoryCommittee/UCM307880.pdf (Accessed June 26, 2024).
9. Joint Meeting of the Arthritis Advisory Committee (AAC) and the Drug Safety and Risk Management Advisory Committee. Meeting transcript. March 25, 2021. Available online at: https://www.fda.gov/media/150663/download (Accessed July 1, 2024).
10. Joint Meeting of the Arthritis Advisory Committee (AAC) and the Drug Safety and Risk Management Advisory Committee. Meeting transcript. March 24, 2021. Available onilne at: https://www.fda.gov/media/150662/download (Accessed July 1, 2024).
11. Roemer, FW, Hochberg, MC, Carrino, JA, Kompel, AJ, Diaz, L, Hayashi, D, et al. Role of imaging for eligibility and safety of a-NGF clinical trials. Ther Adv Musculoskelet Dis. (2023) 15:1–11. doi: 10.1177/1759720X231171768
PubMed Abstract | Crossref Full Text | Google Scholar
12. Gliklich, RE, Dreyer, NA, and Leavy, MB. Registries for evaluating patient outcomes: a User’s guide [internet]. 3rd ed. Rockville (MD): Agency for Healthcare Research and Quality (US) (2014).
13. Dally, A. Thalidomide: was the tragedy preventable? Lancet. (1998) 351:1197–9. doi: 10.1016/S0140-6736(97)09038-7
PubMed Abstract | Crossref Full Text | Google Scholar
14. U.S. Food and Drug Administration. FDA notifies veterinarians about adverse events reported in dogs treated with Librela (bedinvetmab injection). Available online at: https://www.fda.gov/animal-veterinary/product-safety-information/dear-veterinarian-letter-notifying-veterinarians-about-adverse-events-reported-dogs-treated-librela (Accessed January 6, 2025)
15. Woodward, KN. Veterinary pharmacovigilance. Part 5. Causality and expectedness. J Vet Pharmacy Ther. (2005) 28:203–11. doi: 10.1111/j.1365-2885.2005.00649.x
PubMed Abstract | Crossref Full Text | Google Scholar
16. EMA. Veterinary Dictionary for Drug Related Affairs (VeDDRA) list of clinical terms for reporting suspected musculoskeletal AE to veterinary medicinal products. Available online at: http://justusrandolph.net/kappa/ (Accessed October 11, 2024).
17. Groups.io Ortholistserv private forum hosted by groups.io. Librela thread, September 4, 2023.
18. Dimitroulas, T, Lambe, T, Raphael, JH, Kitas, GD, and Duarte, RV. Biologic drugs as analgesics for the management of low back pain and sciatica. Pain Med. (2019) 20:1678–86. doi: 10.1093/pm/pny214
PubMed Abstract | Crossref Full Text | Google Scholar
19. Randolph, JJ. Online Kappa Calculator. Available online at: http://justus.randolph.name/kappa (Accessed September 27, 2024).
20. Sono, T, Meyers, CA, Miller, D, Ding, C, McCarthy, EF, and James, AW. Overlapping features of rapidly progressive osteoarthrosis and Charcot arthropathy. J Orthop. (2019) 16:260–4. doi: 10.1016/j.jor.2019.02.015
PubMed Abstract | Crossref Full Text | Google Scholar
21. Erles, K. Veterinary pathology group, Hitchin, UK. Redacted reports are available on request
22. Zoetis. Zoetis’ statement on the safety of Librela, December 18 2024. Available online at: https://news.zoetis.com/press-releases/press-release-details/2024/Zoetis-Statement-on-the-Safety-of-Librela/default.aspx (Accessed January 6, 2025)
23. Calfas, J. What killed their pets? Owners blame meds, but vets aren’t sure. Wall Street Journal. April 12, 2024. Available online at: https://www.wsj.com/health/pharma/dog-cat-arthritis-drugs-bcdddea6?st=nqd8io6pc74r71z&reflink=desktopwebshare_permalink (Accessed August 10, 2024)
24. European Commission. European Commission opens investigation into possible anticompetitive conduct by Zoetis over novel pain medicine for dogs. Available online at: https://ec.europa.eu/commission/presscorner/detail/en/ip_24_1687 (Accessed March 30, 2024)
25. Hillier, TN, Watt, MM, Grimes, JA, Berg, AN, Heinz, JA, and Dickerson, VM. Dogs receiving cyclooxygenase-2–sparing nonsteroidal anti-inflammatory drugs and/or nonphysiologic steroids are at risk of severe gastrointestinal ulceration. J Am Vet Med Assoc. (2024) 263:1–8. doi: 10.2460/javma.24.06.0430
PubMed Abstract | Crossref Full Text | Google Scholar
26. Innes, JF, Clayton, J, and Lascelles, BD. Review of the safety and efficacy of long-term NSAID use in the treatment of canine osteoarthritis. Vet Rec. (2010) 166:226–30. doi: 10.1136/vr.c97
PubMed Abstract | Crossref Full Text | Google Scholar
27. Hunt, JR, Dean, RS, Davis, GN, and Murrell, JC. An analysis of the relative frequencies of reported adverse events associated with NSAID administration in dogs and cats in the United Kingdom. Vet J. (2015) 206:183–90. doi: 10.1016/j.tvjl.2015.07.025
PubMed Abstract | Crossref Full Text | Google Scholar
28. Jones, GM, Pitsillides, AA, and Meeson, RL. Moving beyond the limits of detection: the past, the present, and the future of diagnostic imaging in canine osteoarthritis. Front Vet Sci. (2022) 9:789898. doi: 10.3389/fvets.2022.789898
PubMed Abstract | Crossref Full Text | Google Scholar
29. Monteiro, BP, Simon, T, Knesl, O, Mandello, K, Nederveld, S, Olby, NJ, et al. Global pharmacovigilance reporting of the first monoclonal antibody for canine osteoarthritis: a case study with bedinvetmab (Librela™). Front Vet Sci. 12:1558222. doi: 10.3389/fvets.2025.1558222
Crossref Full Text | Google Scholar
30. Menges, S, Michaelis, M, and Kleinschmidt-Dörr, K. Anti-NGF treatment worsens subchondral bone and cartilage measures while improving symptoms in floor-housed rabbits with osteoarthritis. Front Physiol. (2023) 14:1201328. doi: 10.3389/fphys.2023.1201328
PubMed Abstract | Crossref Full Text | Google Scholar
31. LaBranche, TP, Bendele, AM, Omura, BC, Gropp, KE, Hurst, SI, Bagi, CM, et al. Nerve growth factor inhibition with tanezumab influences weight-bearing and subsequent cartilage damage in the rat medial meniscal tear model. Ann Rheum Dis. (2017) 76:295–302. doi: 10.1136/annrheumdis-2015-208913
PubMed Abstract | Crossref Full Text | Google Scholar
32. Iff, I, Hohermuth, B, Bass, D, and Bass, M. A case of potential rapidly progressing osteoarthritis (RPOA) in a dog during bedinvetmab treatment. Vet Anaesth Analg. 52:263–5. doi: 10.1016/j.vaa.2024.11.041
PubMed Abstract | Crossref Full Text | Google Scholar
33. Berenbaum, F, Blanco, FJ, Guermazi, A, Miki, K, Yamabe, T, Viktrup, L, et al. Subcutaneous tanezumab for osteoarthritis of the hip or knee: efficacy and safety results from a 24-week randomised phase III study with a 24-week follow-up period. Ann Rheum Dis. (2020) 79:800–10. doi: 10.1136/annrheumdis-2019-216296
PubMed Abstract | Crossref Full Text | Google Scholar
34. deShazo, R, Johnson, M, Eriator, I, and Rodenmeyer, K. Backstories on the U.S. opioid epidemic: good intentions gone bad, an industry gone rogue and watch dogs gone to sleep. Am J Med. (2018) 131:595–601. doi: 10.1016/j.amjmed.2017.12.045
PubMed Abstract | Crossref Full Text | Google Scholar
35. Kronenberger, K. In dogs diagnosed with osteoarthritis, how safe and effective is long-term treatment with bedinvetmab in providing analgesia? Vet Evid. (2023) 8. doi: 10.18849/ve.v8i1.598
Crossref Full Text | Google Scholar
36. Farrell, M, Adams, R, and von Pfeil, D. Re: laboratory safety evaluation of bedinvetmab, a canine anti-nerve growth factor monoclonal antibody, in dogs. Vet J. (2024) 305:106104. doi: 10.1016/j.tvjl.2024.106104
PubMed Abstract | Crossref Full Text | Google Scholar
37. Krautmann, M, Walters, R, Cole, P, Tena, J, Bergeron, LM, Messamore, J, et al. Laboratory safety evaluation of bedinvetmab, a canine anti-nerve growth factor monoclonal antibody, in dogs. Vet J. (2021) 276:105733. doi: 10.1016/j.tvjl.2021.105733
PubMed Abstract | Crossref Full Text | Google Scholar
38. Librela US Datasheet. Available online at: https://www.zoetisus.com/content/_assets/docs/vmips/package-inserts/librela-prescribing-information.pdf (Accessed September 28, 2024).
39. Werts, A, Reece, D, Simon, T, and Cole, P. Re: re: laboratory safety evaluation of bedinvetmab, a canine anti-nerve growth factor monoclonal antibody, in dogs. The Vet J. (2024) 306:106175. doi: 10.1016/j.tvjl.2024.106175
PubMed Abstract | Crossref Full Text | Google Scholar
40. Corral, MJ, Moyaert, H, Fernandes, T, Escalada, M, Tena, JK, Walters, RR, et al. A prospective, randomized, blinded, placebo-controlled multisite clinical study of bedinvetmab, a canine monoclonal antibody targeting nerve growth factor, in dogs with osteoarthritis. Vet Anaesth Analg. (2021) 48:943–55. doi: 10.1016/j.vaa.2021.08.001
PubMed Abstract | Crossref Full Text | Google Scholar
41. Michels, GM, Honsberger, NA, Walters, RR, Tena, JK, and Cleaver, DM. A prospective, randomized, double-blind, placebo-controlled multisite, parallel-group field study in dogs with osteoarthritis conducted in the United States of America evaluating bedinvetmab, a canine anti-nerve growth factor monoclonal antibody. Vet Anaesth Analg. (2023) 50:446–58. doi: 10.1016/j.vaa.2023.06.003
PubMed Abstract | Crossref Full Text | Google Scholar
42. Heinemeier, KM, Schjerling, P, Heinemeier, J, Møller, MB, Krogsgaard, MR, Grum-Schwensen, T, et al. Radiocarbon dating reveals minimal collagen turnover in both healthy and osteoarthritic human cartilage. Sci Transl Med. (2016) 8:346ra90-346ra90. doi: 10.1126/scitranslmed.aad8335
PubMed Abstract | Crossref Full Text | Google Scholar
43. European Medicines Agency. Information flow in the pharmacovigilance system. Available online at: https://www.ema.europa.eu/en/documents/scientific-guideline/vich-gl24-guideline-pharmacovigilance-veterinary-medicinal-products-management-adverse-event-reports-aers_en.pdf (Accessed January 8, 2025).
44. VMD Connect. Available online at: https://www.vmdconnect.uk/adverse-events (Accessed July 11, 2024).
Glossary
ADR – Adverse drug reaction
AE – adverse event
AER – adverse event report
aNGFmAb – anti-NGF monoclonal antibody
CT – computed tomography
EVD – EudraVigilance database
EEBVS – European Board of Veterinary Specialisation
EMA – European Medicines Agency
FDA – Food and Drug Administration
HCF – humeral condylar fracture
HIF – humeral intracondylar fissure
MAH – marketing authorization holder
MRI – magnetic resonance imaging
MCP – medial coronoid process
MSAE – musculoskeletal adverse event
MSAER – musculoskeletal adverse event report
NGF – nerve growth factor
NSAIDs – non-steroidal anti-inflammatory drugs
OA – osteoarthritis
RPOA – rapidly progressive osteoarthritis
REMS – risk evaluation and mitigation strategy
TPLO – tibial plateau leveling osteotomy
VeDDRA – Veterinary Dictionary for Drug-Related Adverse Reactions
VMD – Veterinary Medicines Directorate
Keywords: bedinvetmab, Librela, NGF, rapidly progressive osteoarthritis, RPOA, accelerated joint destruction
Citation: Farrell M, Waibel FWA, Carrera I, Spattini G, Clark L, Adams RJ, Von Pfeil DJF, De Sousa RJR, Villagrà DB, Amengual-Vila M, Paviotti A, Quinn R, Harper J, Clarke SP, Jordan CJ, Hamilton M, Moores AP and Greene MI (2025) Musculoskeletal adverse events in dogs receiving bedinvetmab (Librela). Front. Vet. Sci. 12:1581490. doi: 10.3389/fvets.2025.1581490
Received: 22 February 2025; Accepted: 04 April 2025;
Published: 09 May 2025.
Edited by:Ismael Hernández Avalos, National Autonomous University of Mexico, Mexico
Reviewed by:Tania Perez Jimenez, Washington State University, United States
Agatha Elisa Miranda Cortés, National Autonomous University of Mexico, Mexico
Copyright © 2025 Farrell, Waibel, Carrera, Spattini, Clark, Adams, Von Pfeil, De Sousa, Villagrà, Amengual-Vila, Paviotti, Quinn, Harper, Clarke, Jordan, Hamilton, Moores and Greene. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mike Farrell, vetlessons@gmail.com; Ines Carrera, inescarrerayanez@gmail.com; Louise Clark, louise.clark@vetspecialists.co.uk
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Source: Frontiers in Veterinary Science